We want to hear from you! Take the survey.
How do you use It’s Your Yale? How can it be improved? Answer for a chance to win Yale swag.
Human Research Training
Initial Training Requirements
Initial training requirement can be met by completing one of the following options:
- CITI basic training, available through the Yale Workday Learning website.
- Completion of training from some other training provider, i.e., another University’s training program, DHHS or the FDA. Documentation of completion along with the detailed description of the training must be provided to the IRB office for verification and manual entry into the system.
- Completion of 5 modules of the OHRP Human Research Protection Training. Email the 5 certificates you will receive at the end of each module to irb.training@yale.edu.
Human Subjects Protection Training for Community Partners
CIRTification: Community Involvement in Research Training is a human research protection training program designed especially for community partners. If your research involves community members recruiting research participants, obtaining informed consent, or collecting data, this training is appropriate for you study team members.
Instructions:
- Visit the Center for Clinical and Translational Science (CCTS) Training Center website
- Click “Register” (top right hand corner). Select “I am not from UIC.”
- Complete the registration form. When asked to select a site, select ‘Yale University’.
- Click “Register” to finish.
- Visit the Course Catalog. Information about CIRTification will appear. Click “Learn More” and “Enroll” to enroll in CIRTification.
- Email the certificate of completion to irb.training@yale.edu.
Continuing Education
The continuing education requirement can be met by attending any Human Research Protection Program educational session, or by completing any one of the Yale human research modules, or any one of the CITI continuing education modules available through the Yale Workday Learning website.
You can also complete a module of your choice from the OHRP Human Research Protection Training. Email the certificate you will receive at the end of the module to irb.training@yale.edu.
HIPAA Training
Investigators engaged in human subject research conducted at HIPAA covered entities that involves collection or interaction with PHI, must also complete HIPAA training. HIPAA training modules.
Good Clinical Practice Training
Individuals engaged in the conduct of a clinical trial (per the NIH definition) must complete a Good Clinical Practice (GCP) training. The training must be refreshed every three years. Any of the following will satisfy the initial and/or continuing education GCP training requirement:
- Attendance at the instructor-led GCP training offered by Yale Center for Clinical Investigation (YCCI) or Yale HRPP. The sessions are offered several times a year. Registration is available through the Yale Workday Learning website. Sign up for the HRPP newsletter to be notified about the upcoming sessions.
- Completion of the industry-sponsored GCP training conducted by the TransCelerate identified provider. The certificates should be emailed to irb.training@yale.edu for data entry.
- Completion of the online training. The links to the online training sessions are available in the Yale Workday Learning website.
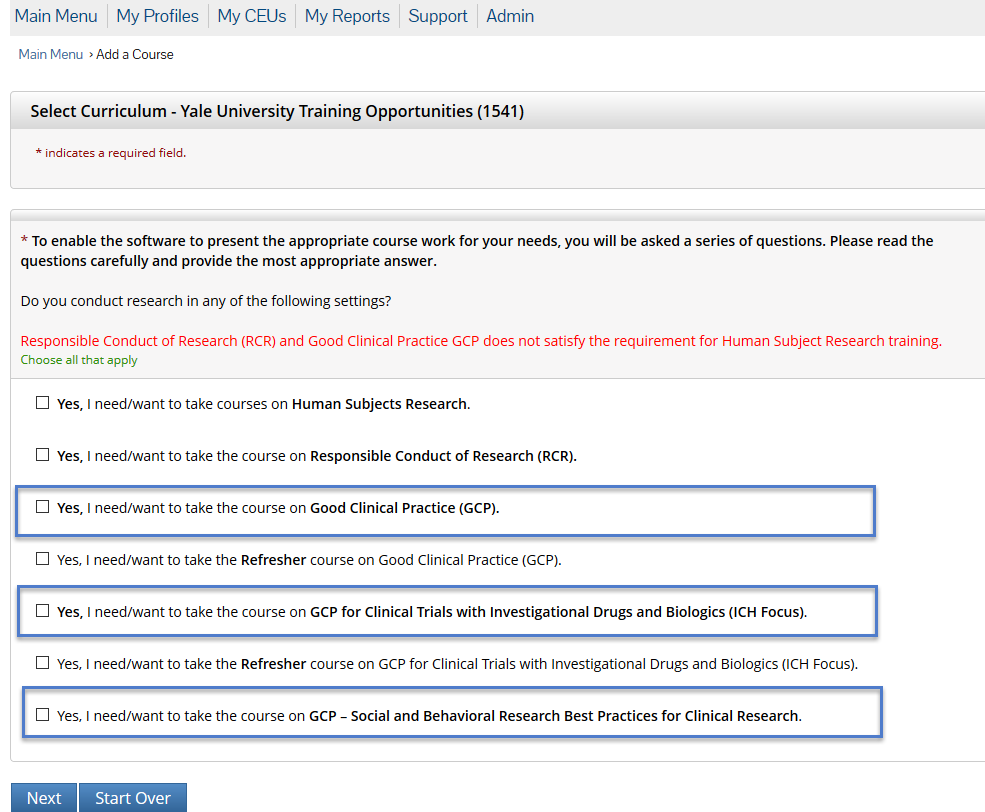
- Completion of any single GCP course through CITI. Once you log in, add a new course from the Main Menu. You will be offered different courses in the Yale portfolio. Be advised that Human Subject Research and Responsible Conduct of Research courses do not satisfy the GCP training requirement. See below for a screenshot of the available GCP courses. CITI notifies the HRPP Office when a course has been successfully completed (all modules within a course must be completed). Workday Learning and IRES IRB records are updated within 1 to 2 business days.
- Please, see the guide for step-by-step instructions on how to complete CITI GCP training.
ESCRO Training Requirements
All Yale University and affiliate individuals, including faculty, staff, postdoctoral scholars, students, visiting scholars and other researchers who plan to engage in Human Embryonic Stem Cell (hESC) research or Induced Pluripotent Stem Cell (iPSC) research that requires Embryonic Stem Cell Research Oversight (ESCRO) are required to satisfactorily complete training regarding the ethical considerations and the policies and regulations pertaining to hESC Research or covered iPS research before beginning preparatory work on any project involving hESCs or iPS cells.
Initial training requirements can be met by completing the following CITI course and related modules:
- Biomedical Research – Basic/Refresher Course (which includes the two required modules below):
- Stem Cell Research Oversight (Part I)
- Stem Cell Research Oversight (Part II)
Please email your Completion Report (transcript showing the completion of the two stem cell research modules) to irb.training@yale.edu.


