We want to hear from you! Take the survey.
How do you use It’s Your Yale? How can it be improved? Answer for a chance to win Yale swag.
External IRBs
Yale HRPP may authorize use of an external IRB for review of certain types of research studies. The categories of research eligible for external IRB review are described in the HRPP 920 Procedure 4 titled ‘Use of External IRBs for Review and Oversight of Research Involving Human Subjects’.
The links to the information about the specific external IRBs available for use are included in the menu tab on the left hand side.
Note that when an external IRB reviews the study on behalf of Yale, only the actual IRB review is ceded to that IRB. The local requirements regarding research will remain the institutional responsibility. As such, the Principal Investigator will continue working with the Yale HRPP to ensure that investigators and research staff meet the Yale training and COI disclosure requirements, all ancillary reviews (e.g. PPRC, PRC, MRRC) are obtained, the record is updated in IRES IRB etc.
Process of Obtaining Authorization to Use External IRB
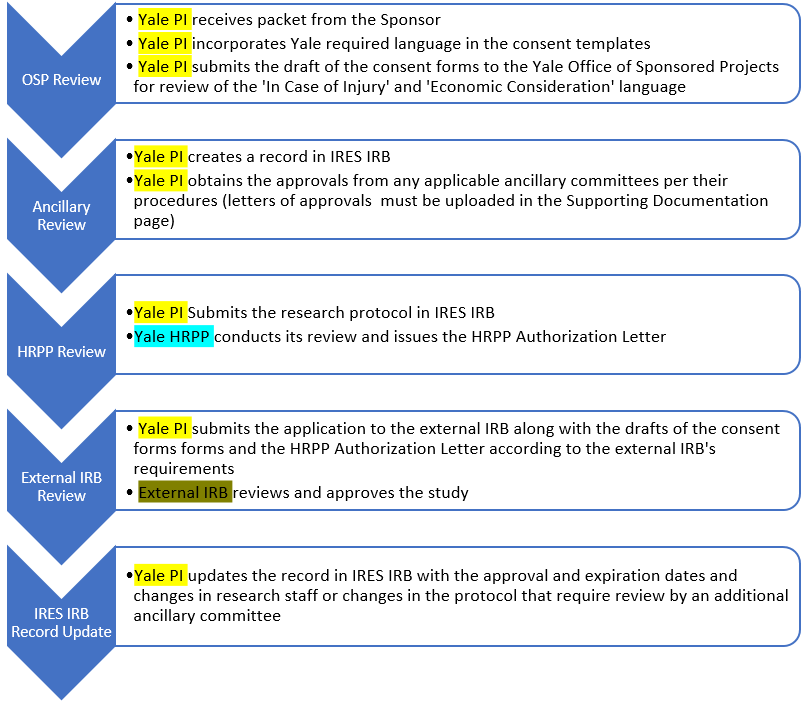
PI’s Post-Approval Responsibilities
- Once approved as a site by the external IRB, update the study record in IRES IRB to provide the current expiration date of research and upload the approval letter.
- Keep a regulatory binder and maintain an active record of all submissions to the IRB of record.
- Submit any modifications to the Yale HRPP through IRES IRB that require local review. Examples of such changes include:
- Personnel changes
- Changes of PI
- Changes in conflicts of interest
- Changes for which there is a specific institutional policy/state law requirement e.g. review by an ancillary committee
- Changes to the consent form that require amendment to the sponsor protocol e.g. Economic Considerations, In Case of Injury language sections in the consent form.
- Update the study record in IRES IRB with the new expiration date and approval of continuing review as issued by the external IRB.
- Promptly report to the Yale HRPP any determinations of serious or continuing noncompliance or UPIRSOs that the external IRB made for the Yale site.
- Promptly report to the Yale HRPP any notifications of suspension or termination that you receive for the study from the external IRB.
- Update the IRES IRB when the study was completed and closed by the IRB of record.


